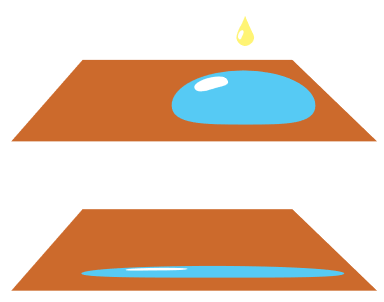
Have you ever seen a bead of water sitting on a surface? This is because water has a property called surface tension. This tension causes water to form a bead on the surface of things like glass or fabric. You can see surface tension at work by placing a drop of water onto a counter top. The drop will hold its shape and will not spread.
In order to clean the dirt on our clothes, the water needs to be able to reach the surface. Water is able to get to the surface if surface tension is reduced. To do this, we use a group of chemicals called surface active agents, or surfactants.
What is a surfactant?
Surfactants change how water behaves. When a surfactant is added, the surface tension is reduced. Now water can spread out and wet the surface (e.g., clothes, dishes, counter tops) we are trying to clean.
Now let’s look at what happens on the surface.
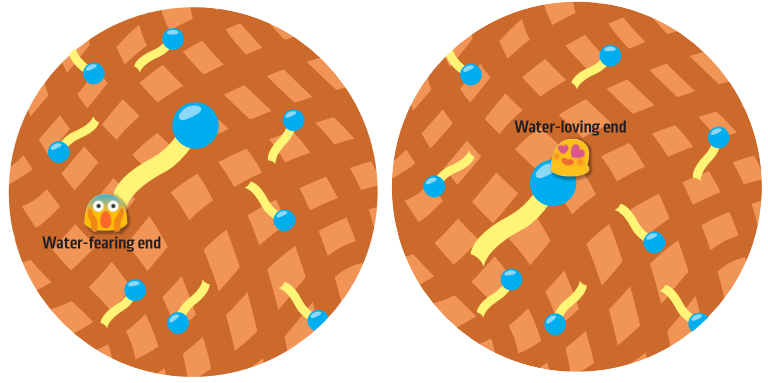
Every surfactant has two ends. One end wants to be in water and the other does not.
The water-fearing end is known as the hydrophobic end. Hydrophobic comes from two Greek roots, hydro- (meaning water) and -phobia (meaning fearing). Have you heard the phrase “oil and water don’t mix?” This is important here!
The water-fearing end of the surfactant is made up of hydrocarbon chains. A hydrocarbon is a molecule that is made of hydrogen and carbon. The chains love oil and grease and will try to stay away from water.
The water-loving end is known as the hydrophilic end. We learned hydro- is a Greek root meaning ‘water’. So, if -phobic means ‘fearing’, then -philic means loving. The water-loving end of the chemical is attracted to water.
How these two ends interact with soil and water is the secret to how a surfactant works.
How Surfactants Clean
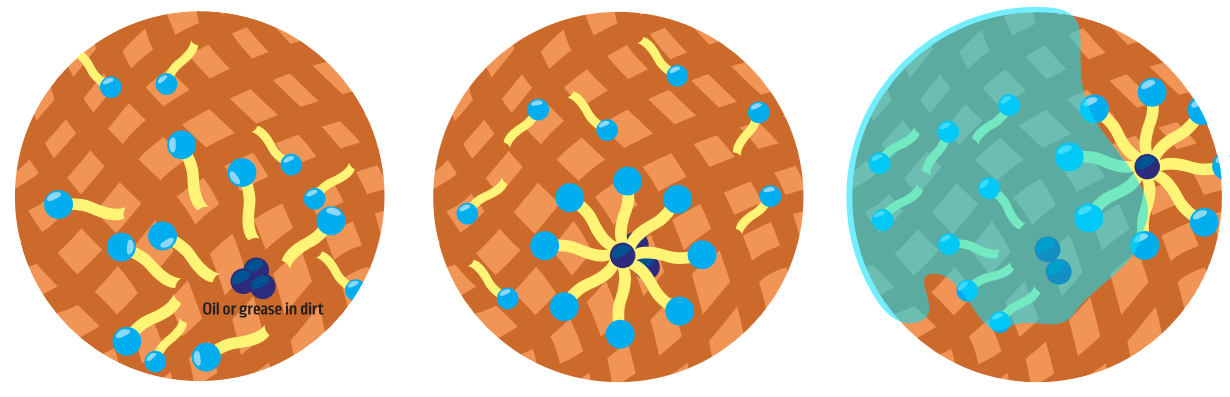
Once the surfactant is added to water, the water-fearing ends try to stay away from the water. They do this by organizing into the shape of a sphere with the water-loving ends on the outside and the water-fearing ends protected on the inside. This spherical shape of surfactants is called a micelle.

The micelle is important because it is what traps the soil. Remember, the inside of the micelle is hydrophobic and does not want to be near water. The soil is also hydrophobic, so it likes the environment the micelle creates.
The attraction of the soil to the inside of the surfactant micelle helps loosen the soil from its surface. Once the soil lifts off the surface, it becomes suspended in the water in the micelle. This suspension is also known as emulsification of one liquid into another. Happy inside the micelle, the soil will not settle back onto the surface.
Now that the soil is trapped in the micelle and the micelle is suspended in water, it is easy to wash the soil way.
Remember the outside of our micelle loves water. So, as we rinse, the micelle floats away and we are left with a clean surface!
Learn More About the Science of Cleaning at ExplorationClean.org